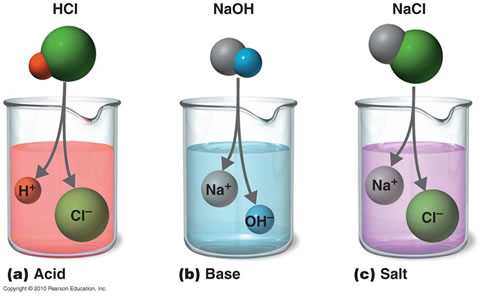
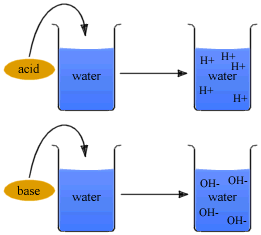
Preparation of Acids and Bases

| Acids | Bases(Oxides /Hydroxides) |
|---|---|
| Hydrogen + non-metal -> Acid H2 + Cl2 -> 2HCl |
Metal + Oxygen -> Base[basic oxide] 2Mg + O2 -> 2MgO |
Acids from Acidic OxidesAcidic oxide + Water -> AcidSO2 + H2O -> H2SO3 |
Bases from Basic OxidesBasic oxide + water -> Base2Na + 2H2O -> 2NaOH + H2 |
Acids from SaltsNormal +Sulphuric -> Acid +DisplacedSalt acid[conc.] salt volatile acid NaCl + H2SO4 <200oC> NaHSO4 + HCl |
Bases from SaltsSalt + Base -> Normal + PrecipitatedSoln. [alkali] Salt basic hydroxide FeSO4 +2NaOH -> Na2SO4 +Fe(OH)2 ↓ |
Acids by oxidation of non-metalsSulphur + Nitric -> Suplhuric + water + nitrogenacid acid dioxide |
Bases by decomposition of saltsLead nitrate -> Lead + nitrogen + oxygenOxide dioxide 2Pb(NO3)2 -> 2PbO + 4NO2 + O2 |