| Acids |
Bases |
|
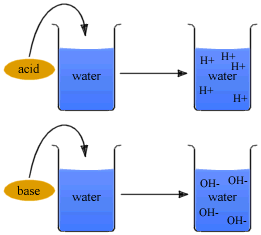
Strength of an acid depends on the concentration of the hydronium ions[H3O+] present in an aqueous solution of an acid.
|
Strength of an Alkali depends on the concentration of hydroxyl ions[OH-] present in an aqueous solution of the alkali.
|
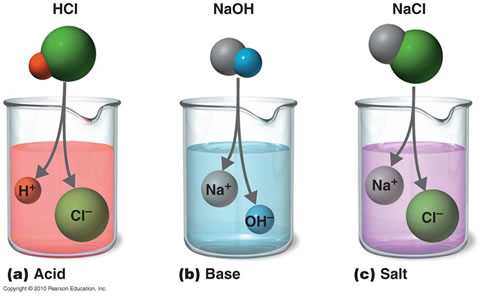
| Strong Acid is one which dissociates almost completely in aqueous solution producing a high concentration of hydrogen[H+] ions[or H3O+] ions].
HNO3 + H2O ↔ H3O+ + NO3-
Examples: Hydrochloric, sulphuric & nitric acid.
|
Strong Alkali is one which dissociates almost completely in aqueous solution producing a high concentration of hydroxyl [OH-] ions.
NaOH ↔ Na+ + OH-
Examples: Lithium, Sodium & potassium hydroxide.
|
| Weak Acid is one which dissociates only partially in aqueous solution.
CH3COOH ↔ CH3COO- + H+
Examples: Acetic acid, Citric acid and Carbonic acid.
|
Weak Alkali is one which dissociates only partially in aqueous solution.
NH4OH[aq] ↔ NH4+ + OH-
Examples: Ammonium and Calcium hydroxide.
|
| Basicity of Acid is the no of hydrogen ions [H+] which can be produced per molecule of the acid in aqueous solution.
Monobasic acid(HCl, HNO3) ionizes in aqueous solution to produce one hydrogen ion per molecule of the acid, Dibasic acid(H2SO3, H2SO4) produces two and Tribasic acid(H3PO4) three.
|
Acidity of Base is the no of hydroxyl ions [OH-] which can be produced per molecule of the base.
Monoacidic base(NaOH, KOH) ionizes in aqueous solution to produce one hydroxyl ion per molecule of the base, Diacidic base(Ca(OH)2, Zn(OH)2) produces two and Triacidic base( Al(OH)3) three.
|