Chemistry
Uses of Acids and Bases
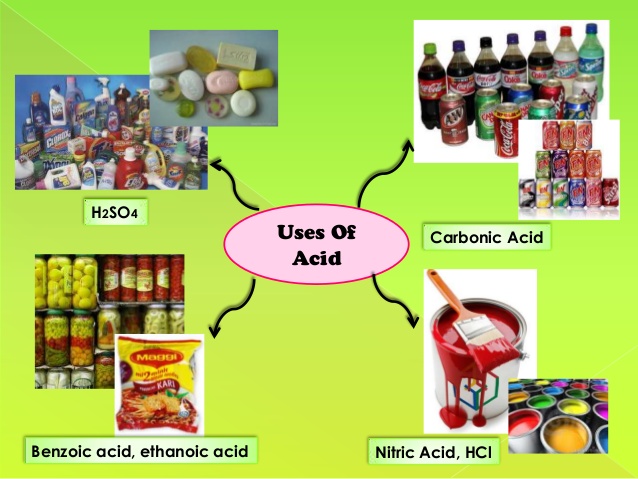
Manufacture of soaps, Manufacture of bleaching powder, Food preservation, Baking powder, Cooking
Read MorePhysical and Chemical Properties of Acids and Bases

Acids neutralize bases to give salt and water only | Alkalis react with ammonium salts on heating to liberate ammonia.
Read MorePreparation of Acids and Bases

Acids from Salts: Normal + Sulphuric -> Acid +Displaced Salt acid[conc.] salt volatile acid NaCl + H2SO4 NaHSO4 + HCl
Read MoreDifference Between Acids and Bases
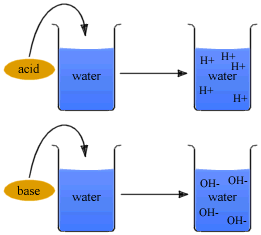
Strength of an acid depends on the concentration of the hydronium ions[H3O+] present in an aqueous solution of an acid. Strength of an Alkali depends on the concentration of hydroxyl ions[OH-] present in an aqueous solution of the alkali.
Read MoreBasics of Acids, Bases and Salts
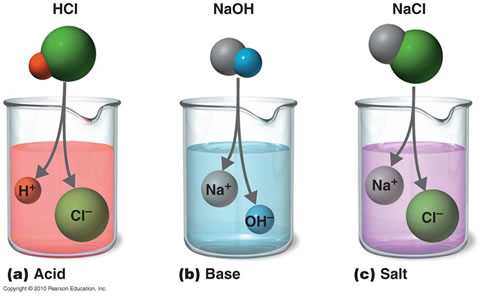
Strength of an acid depends on the concentration of the hydronium ions[H3O+] present in an aqueous solution of an acid. Strength of an Alkali depends on the concentration of hydroxyl ions[OH-] present in an aqueous solution of the alkali.
Read More